Why Choose CroFab?
CroFab Is the Only Antivenom Derived From Native US Pit Vipers to Treat Envenomations From All Species of North American Pit Vipers1
CroFab Meets WHO Guidelines for Effective Snake Antivenom2
According to the World Health Organization (WHO), an effective antivenom is one derived from clinically relevant snakes native to the geographic region in which the antivenom is used.2
CroFab is the only antivenom derived from geographically and clinically relevant US snakes for comprehensive coverage of all North American pit viper envenomations.1,2
CroFab Was Engineered to Neutralize a Broad Range of Clinically Relevant Toxins1
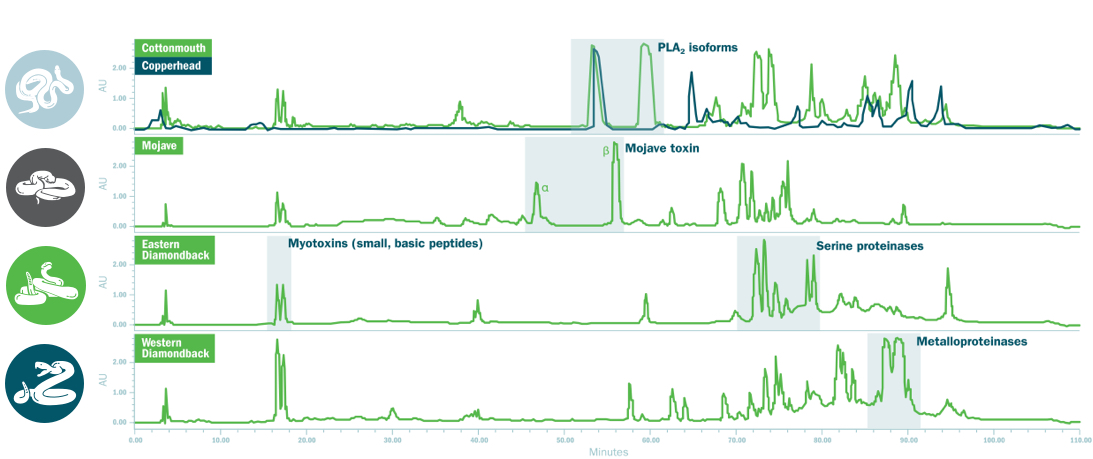
A Comprehensive Venomics Approach
Four snake species were strategically chosen based on their unique venom fingerprints, which together provide comprehensive coverage across the diverse venom compositions of North American pit vipers.1,3
High-performance liquid chromatography is used to separate and identify individual components of venom to define a unique venomic fingerprint for each snake type.4,5
Hear why Dr. Thomas Arnold and Dr. William Banner choose CroFab.
Hear how CroFab helped real patients.
Jessica
Caitlyn
CroFab is clinically proven to achieve initial control of envenomation2
CroFab rapidly binds to and neutralizes venom toxins2
CroFab safety is backed by more than 20 years of clinical experience2



