Mechanism of Action
CroFab Rapidly Binds to and Neutralizes Venom Toxins1
Watch this video to see how CroFab works.
Upon Administration, CroFab Gets to Work
CroFab is specifically designed to contain a spectrum of venom-specific Fab fragments targeting the range of complex toxins found in North American pit viper venoms.1

Envenomation
North American pit viper venom contains a complex array of toxins that can result in a range of local, systemic, and/or hematologic effects2,3
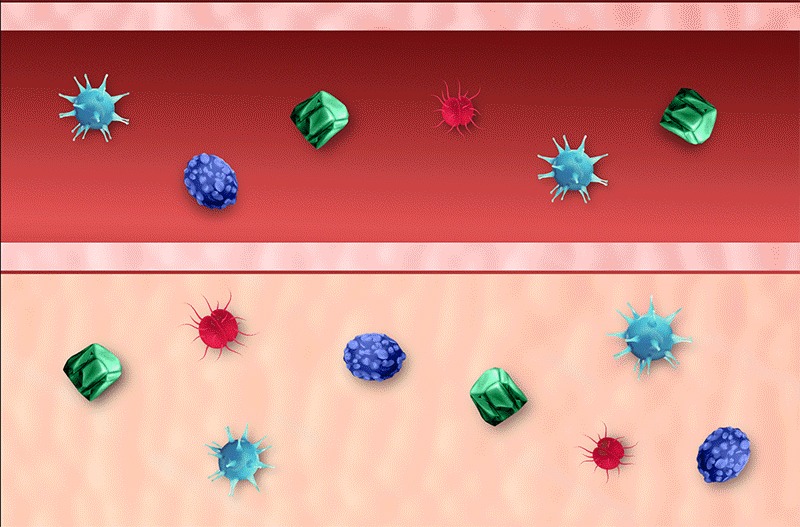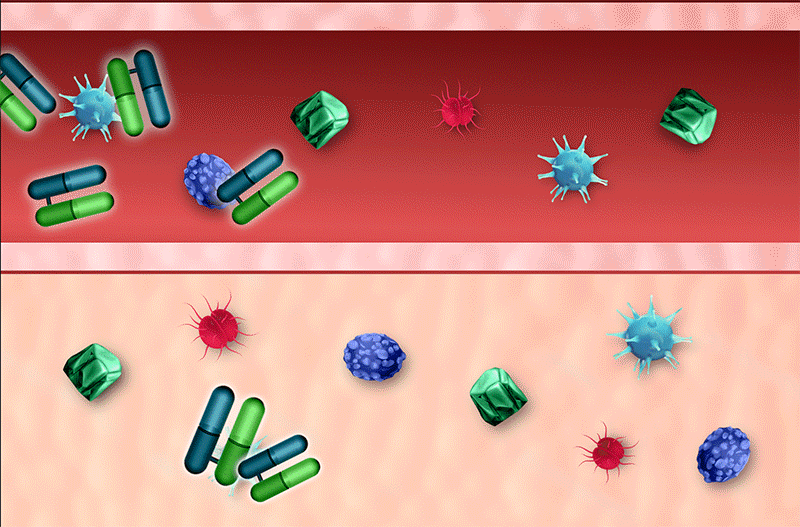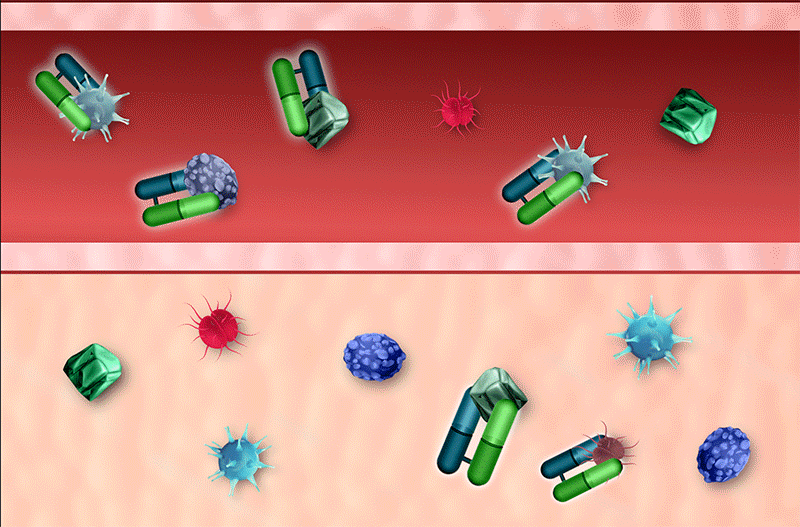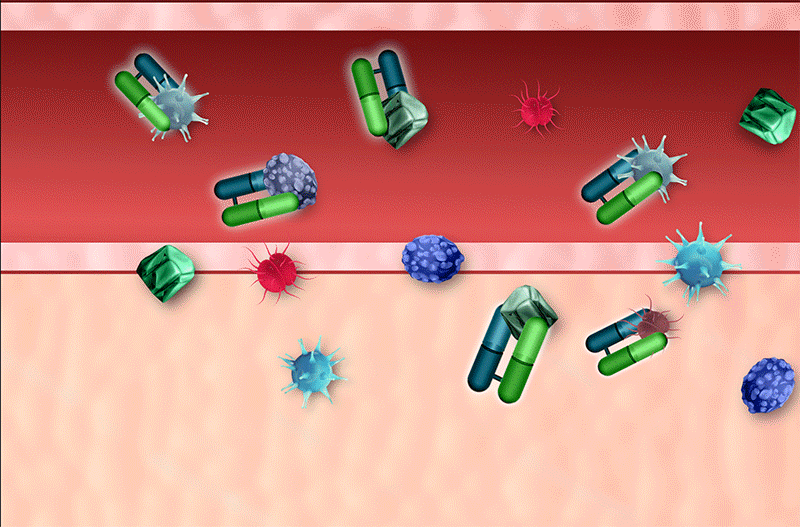
Rapid Neutralization
The small size of venom-specific protein (Fab) fragments permits a rapid, large volume of distribution into tissue, allowing faster neutralization of venom toxins4

Rapid Clearance
Small protein (Fab) fragments also permit faster clearance, allowing for decreased immune system recognition and low incidence of hypersensitivity4
CroFab is clinically proven to achieve initial control of envenomation2
A proven manufacturing process ensures only the most effective and highest quality venom-specific antibodies1,5
Appropriate dosing achieves initial and sustained control of envenomation1