Efficacy of CroFab
CroFab Is Clinically Proven to Arrest Local Injury, Resolve Systemic Effects, and Reduce Hematologic Effects of Envenomation1
Clinically Proven to Achieve Initial and Clinically Relevant Control of North American Pit Viper Envenomation1
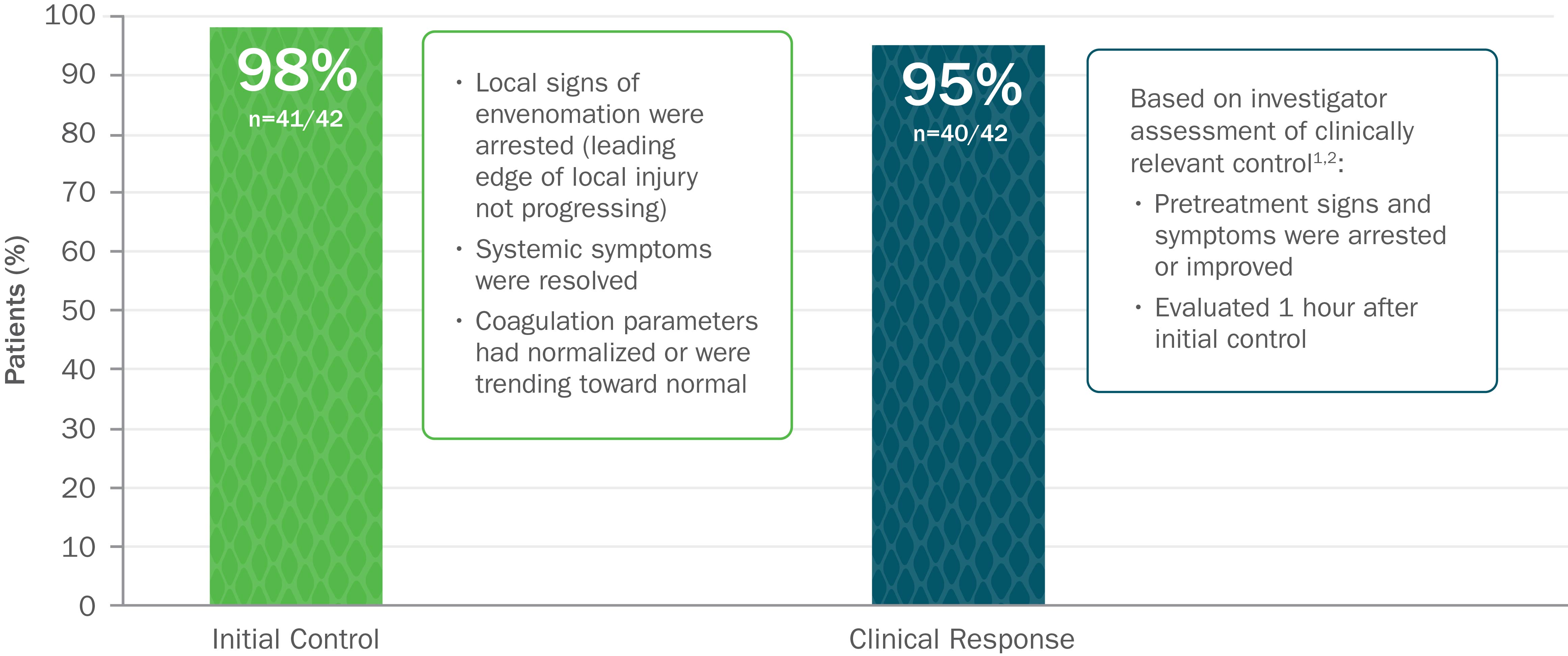
Based on 2 open-label trials with 42 patients with mild or moderate envenomation. Exclusion criteria for both trials included envenomation by copperhead snakes. In study 1, patients were given up to 2 doses of 4 vials each to gain initial control. In study 2, patients were given up to 2 doses of 6 vials each to gain initial control.1
Level 1 Evidence Specifically for Copperhead Envenomation3
CroFab Administration Resulted in Improved Limb Function Following Copperhead Envenomation3
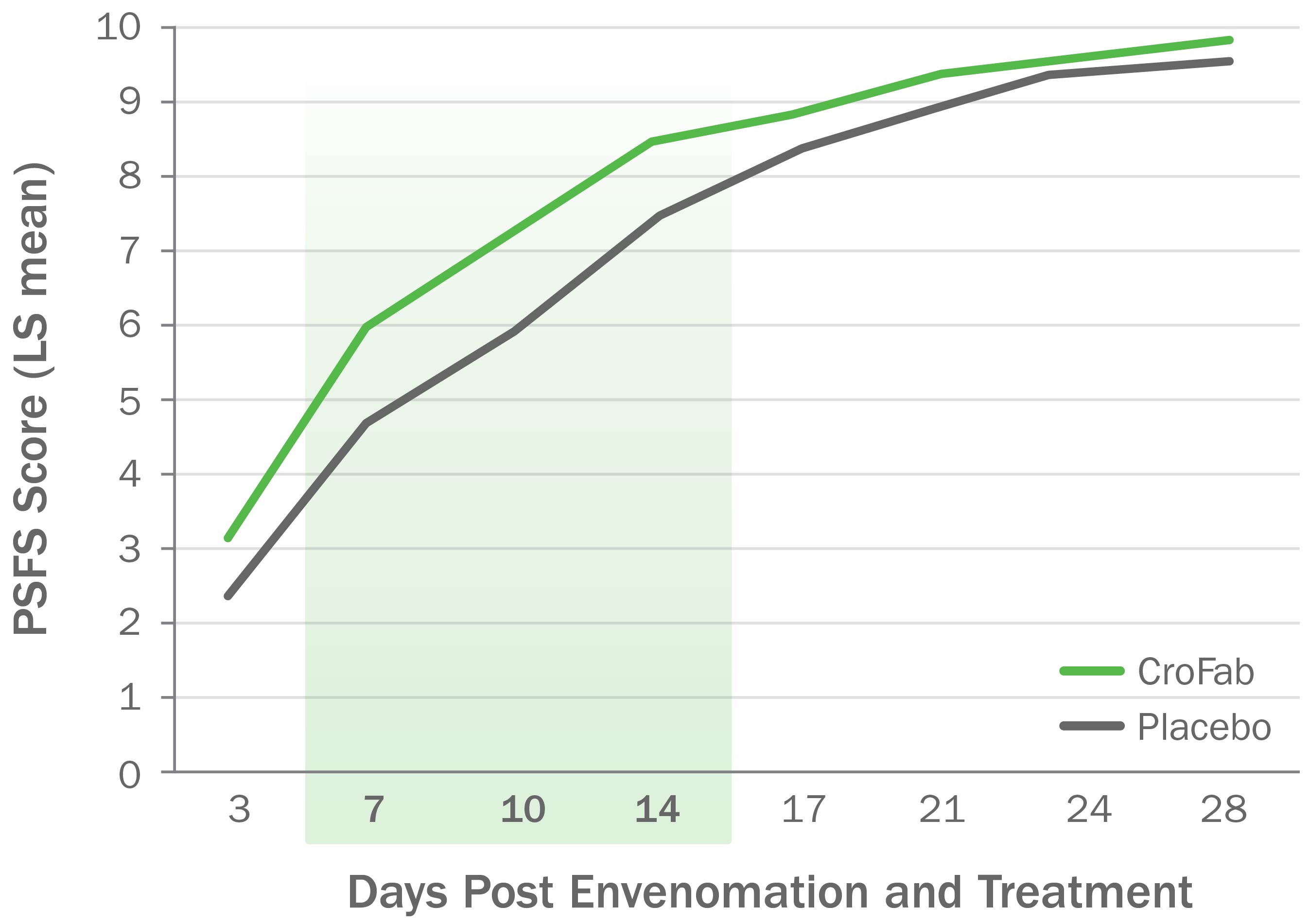
Source: Gerardo CJ et al. Ann Emerg Med. 2017;70(2):233-244.e3.
Clinically meaningful and statistically significant improvements in limb function at 7, 10, and 14 days following envenomation compared with placebo (P<0.05) for all 3 time points).3,4
PSFS, Patient-Specific Functional Scale.
Based on a double-blind, placebo-controlled trial with 74 patients with mild or moderate envenomation.
Patients with severe envenomation were excluded. Patients were given up to 2 doses of 6 vials each to gain initial control.3
Early Administration of CroFab Resulted in Shorter Time to Complete Recovery5
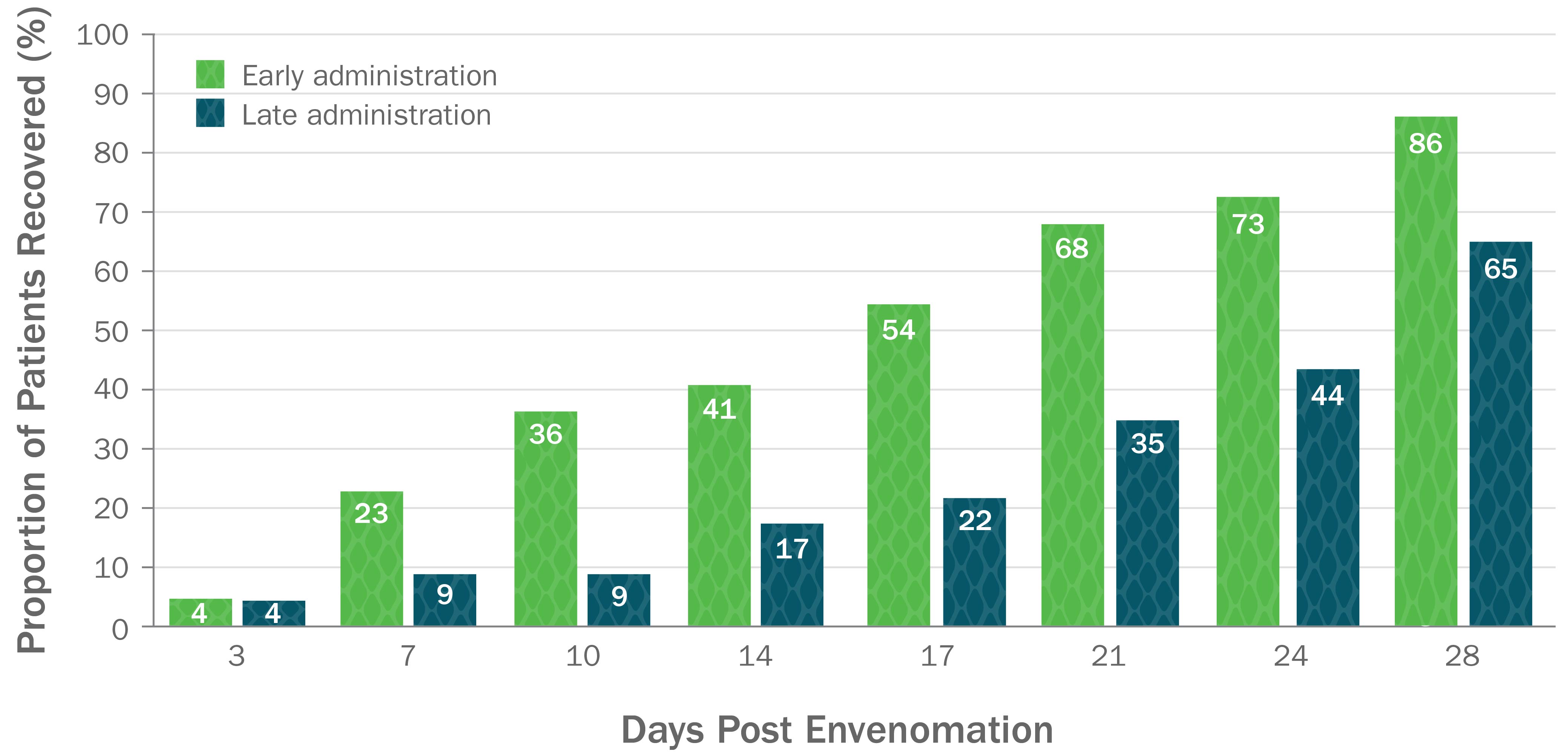
Early administration of CroFab resulted in significantly shorter median time to full recovery compared with later treatment (17 vs 28 days respectively, P=0.025).
Based on a secondary analysis of a double-blind, placebo-controlled trial with 45 patients with mild or moderate envenomation. Patients with severe envenomation were excluded. Patients were given up to 6 vials each to gain initial control. Early treatment was defined as treatment within 5.47 hours post envenomation, and later treatment was defined as treatment that occurred 5.47 or more hours post envenomation.
CroFab Administration Resulted in Less Opioid Use3,*
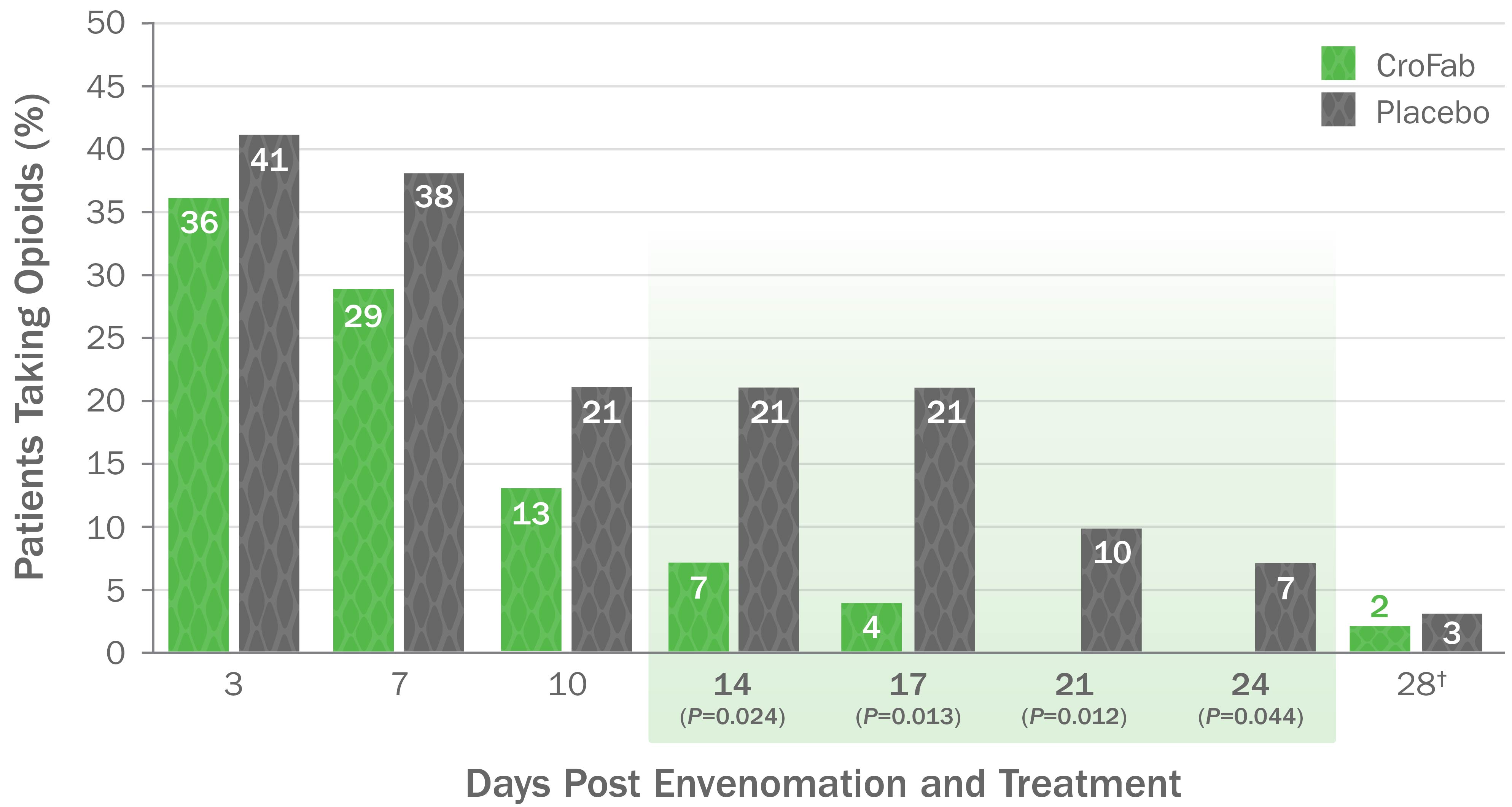
- Treatment with CroFab was associated with less opioid use at all time points (up to 28 days post treatment)3
- Patients treated with CroFab discontinued opioid use by 3 weeks compared with 3 months for untreated patients4
Patients who received placebo were over 5 times more likely to require opioids than those who received CroFab6,‡
*Based on a double-blind, placebo-controlled trial with 74 patients with mild or moderate envenomation. Patients with severe envenomation were excluded. Patients were given up to 2 doses of 6 vials each to gain initial control.
†On Day 28 post envenomation, 1 patient receiving CroFab continued to take a daily opioid prescribed for anxiety prior to study initiation.4
‡Based on a secondary analysis of opioid use post discharge. Two patients were excluded from the secondary analysis.6
Watch Dr. Rutherfoord Rose discuss using CroFab to treat copperhead envenomation.
CroFab Clinical Studies
Review publications evaluating efficacy and safety for CroFab
CroFab safety is backed by more than 20 years of clinical experience1
Appropriate dosing achieves initial and sustained control of envenomation1
Real-world use supports improved outcomes with CroFab1