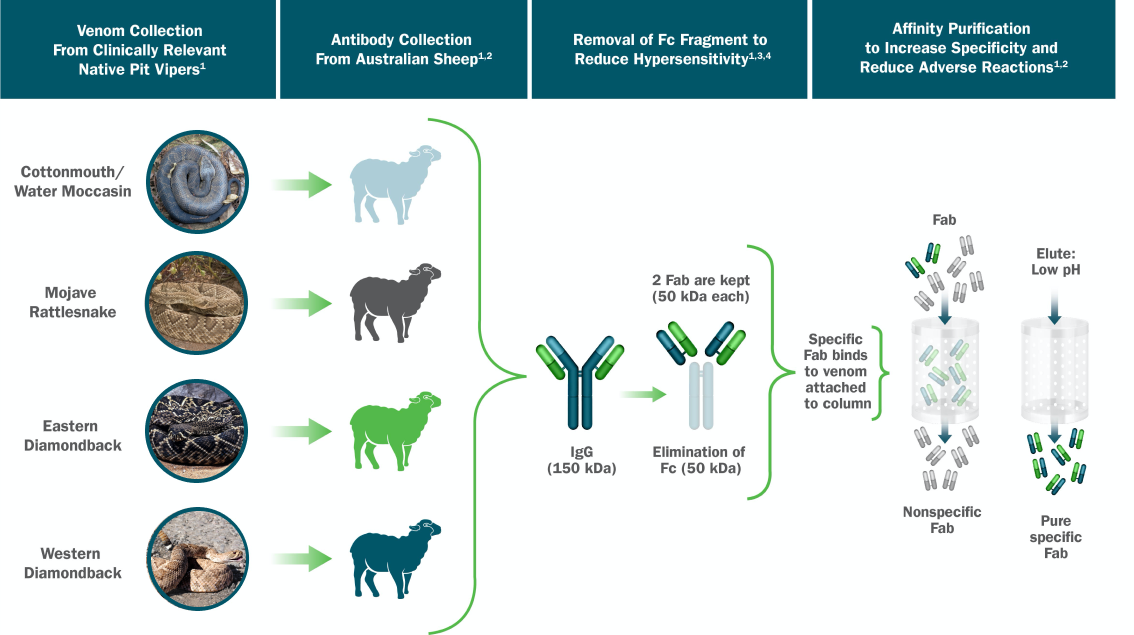
Contraindications
Do not administer CroFab® to patients with a known history of hypersensitivity to any of its components, or to papaya or papain unless the benefits outweigh the risks and appropriate management for anaphylactic reactions is readily available.
Warnings and Precautions
Coagulopathy: In clinical trials, recurrent coagulopathy (the return of a coagulation abnormality after it has been successfully treated with antivenin), characterized by decreased fibrinogen, decreased platelets, and elevated prothrombin time, occurred in approximately half of the patients studied; one patient required re-hospitalization and additional antivenin administration. Recurrent coagulopathy may persist for 1 to 2 weeks or more. Patients who experience coagulopathy due to snakebite should be monitored for recurrent coagulopathy for up to 1 week or longer. During this period, the physician should carefully assess the need for re-treatment with CroFab® and use of any type of anticoagulant or anti-platelet drug.
Hypersensitivity Reactions: Severe hypersensitivity reactions may occur with CroFab®. In case of acute hypersensitivity reactions, including anaphylaxis and anaphylactoid reactions, discontinue infusion and institute appropriate emergency treatment. Patients allergic to papain, chymopapain, other papaya extracts, or the pineapple enzyme bromelain may also have an allergic reaction to CroFab®. Follow-up all patients for signs and symptoms of delayed allergic reactions or serum sickness (e.g., rash, fever, myalgia, arthralgia).
Adverse Reactions
The most common adverse reactions (incidence ≥ 5% of subjects) reported in the clinical studies were urticaria, rash, nausea, pruritus and back pain. Adverse reactions involving the skin and appendages (primarily rash, urticaria, and pruritus) were reported in 12 of the 42 patients. Two patients had a severe allergic reaction (severe hives and a severe rash and pruritus) following treatment and one patient discontinued CroFab® due to an allergic reaction. Recurrent coagulopathy due to envenomation and requiring additional treatment may occur.
Please see full Prescribing Information.