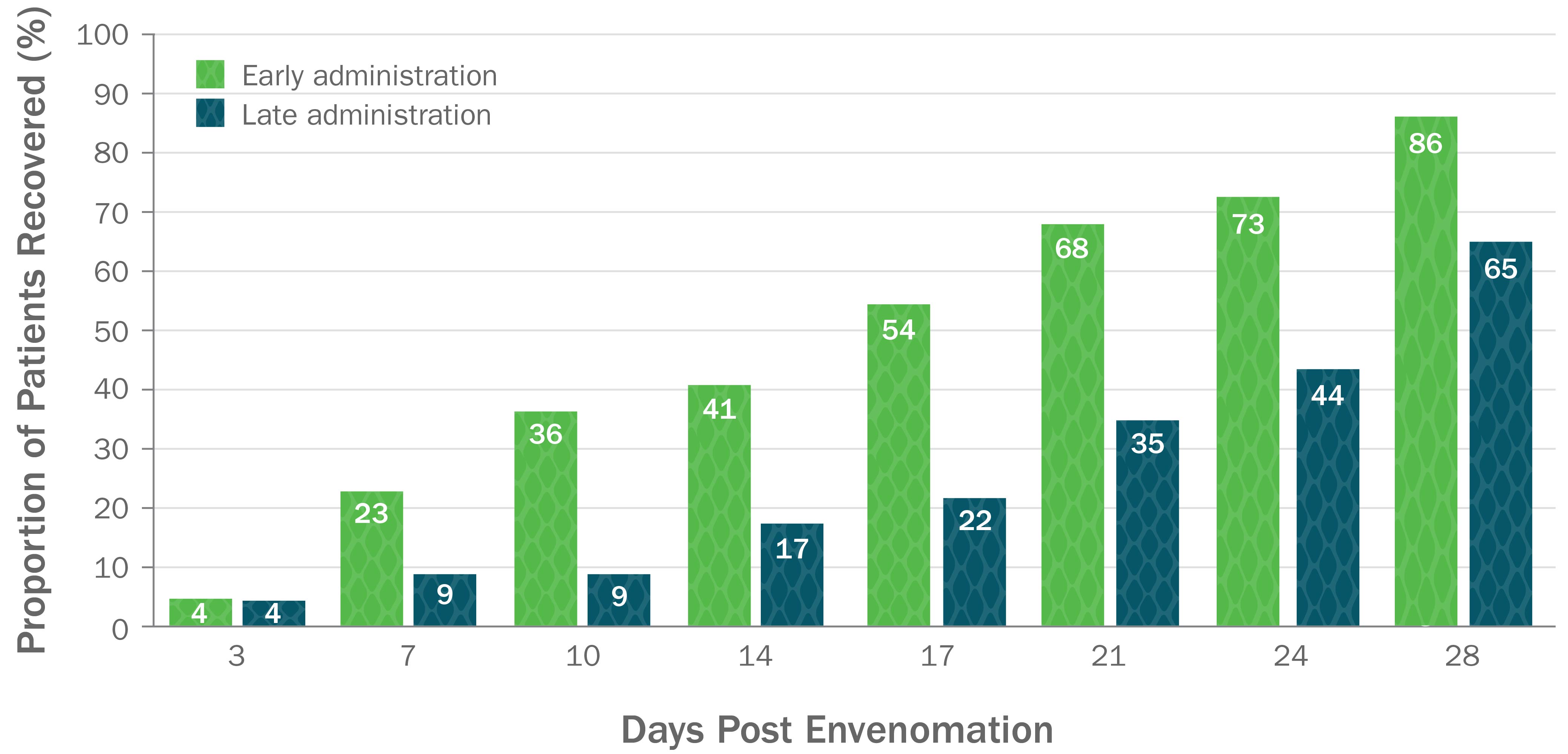
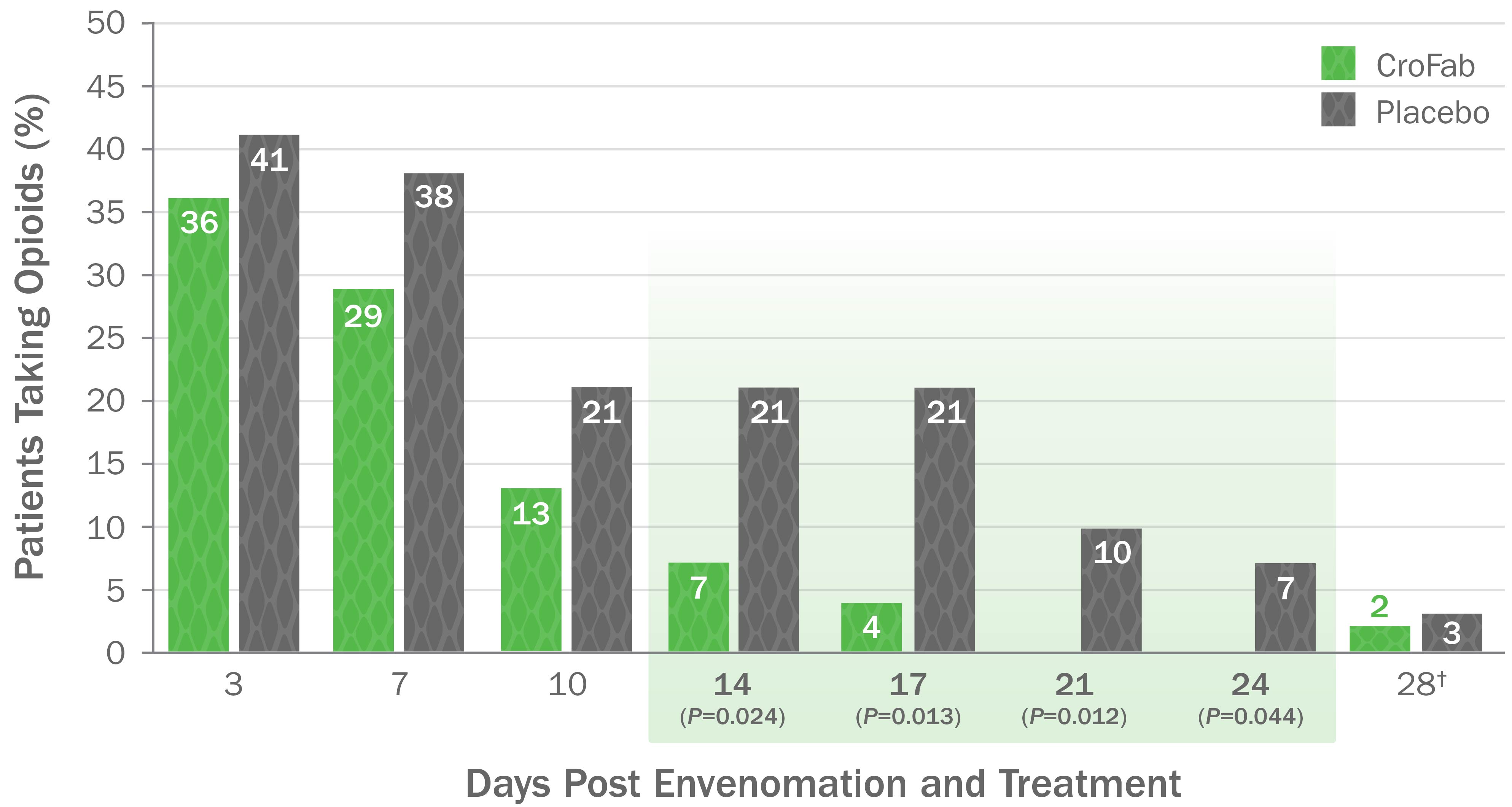
CroFab Is Clinically Proven to Arrest Local Injury, Resolve Systemic Effects, and Reduce Hematologic Effects of Envenomation1
Clinically Proven to Achieve Initial and Clinically Relevant Control of North American Pit Viper Envenomation1
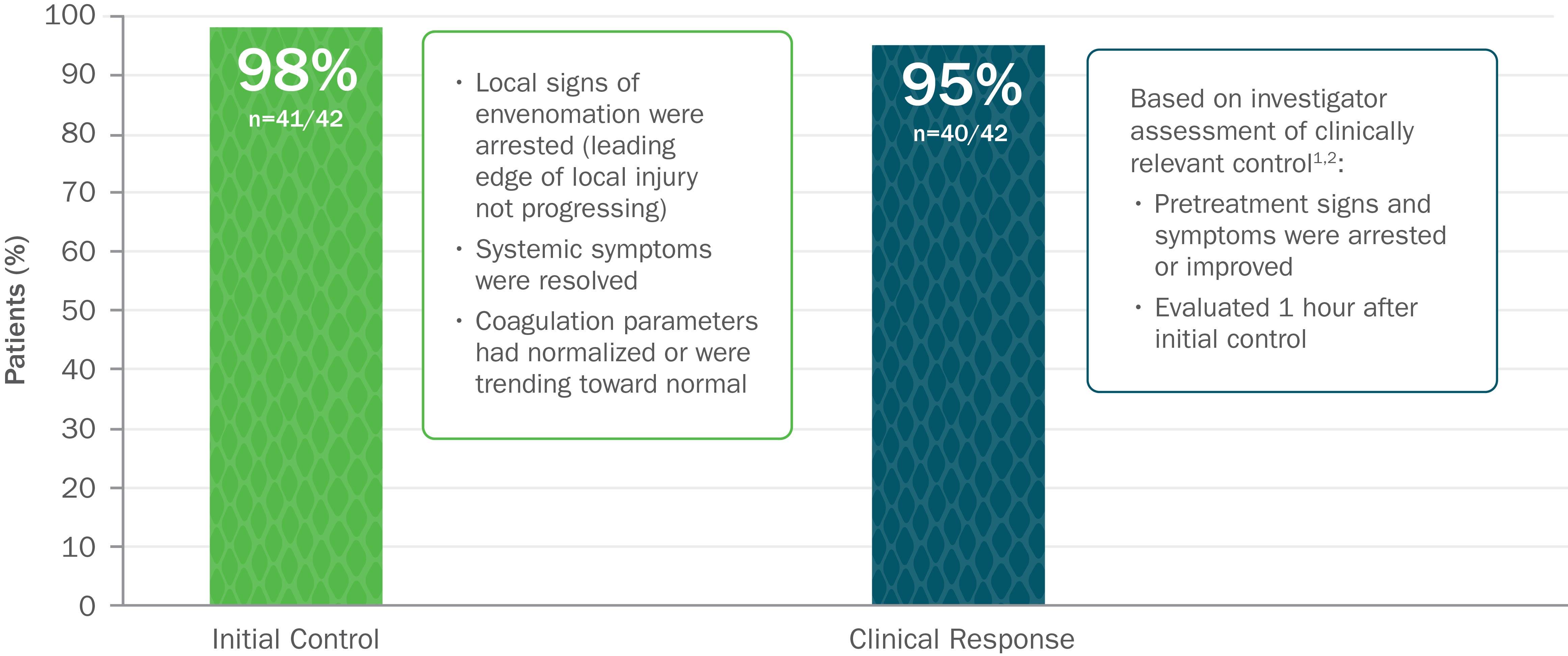
Based on 2 open-label trials with 42 patients with mild or moderate envenomation. Exclusion criteria for both trials included envenomation by copperhead snakes. In study 1, patients were given up to 2 doses of 4 vials each to gain initial control. In study 2, patients were given up to 2 doses of 6 vials each to gain initial control.1
Level 1 Evidence Specifically for Copperhead Envenomation3
CroFab Administration Resulted in Improved Limb Function Following Copperhead Envenomation3
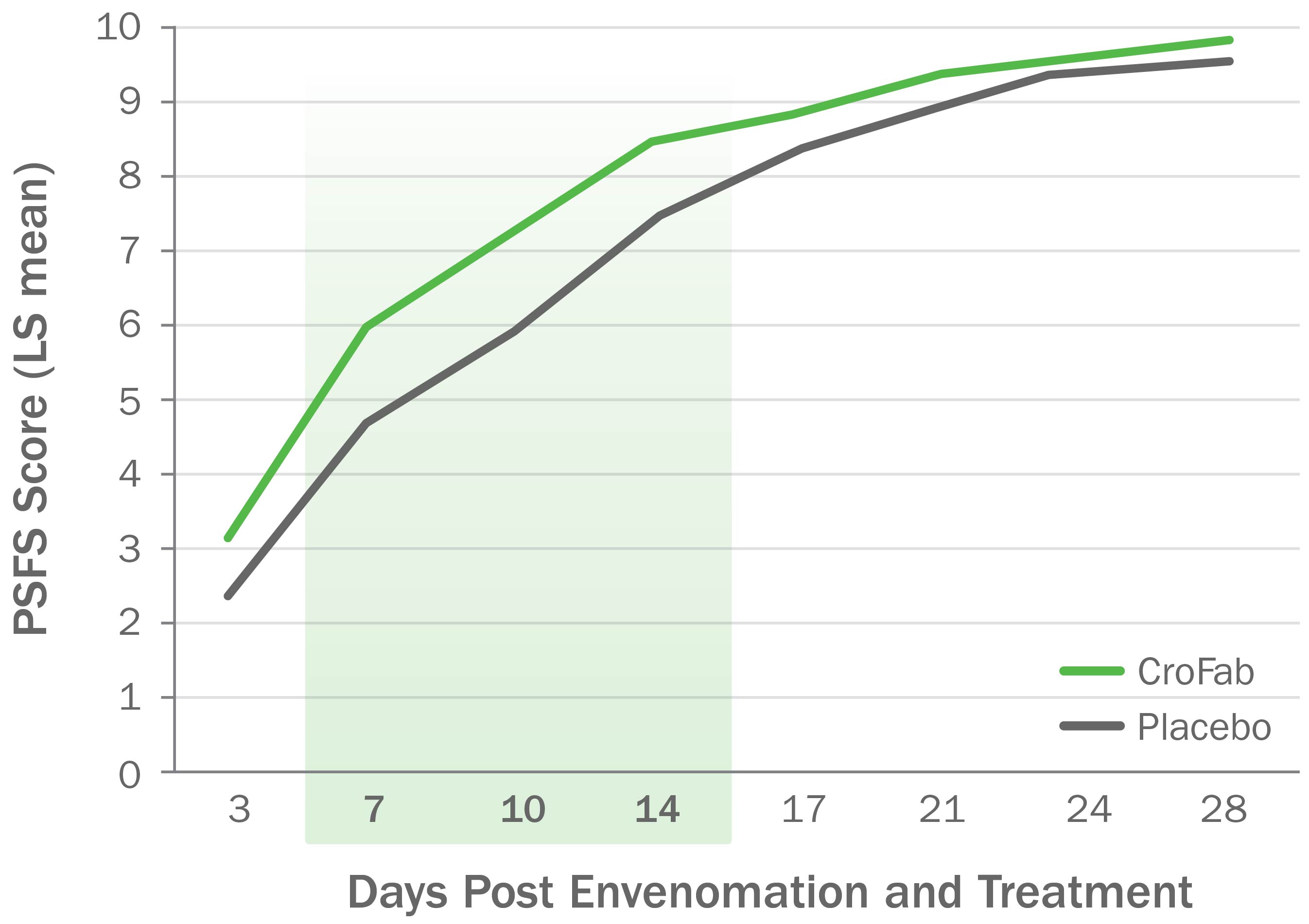
Source: Gerardo CJ et al. Ann Emerg Med. 2017;70(2):233-244.e3.
Clinically meaningful and statistically significant improvements in limb function at 7, 10, and 14 days following envenomation compared with placebo (P<0.05) for all 3 time points).3,4
PSFS, Patient-Specific Functional Scale.
Based on a double-blind, placebo-controlled trial with 74 patients with mild or moderate envenomation.
Patients with severe envenomation were excluded. Patients were given up to 2 doses of 6 vials each to gain initial control.3
REFERENCES
1. CroFab®. Prescribing information. BTG International Inc.; August 2018. 2. WHO Expert Committee on Biological Standardization. WHO Technical Report Series, No. 1004, Annex 5; 2017. Accessed September 13, 2023. https://cdn.who.int/media/docs/default-source/biologicals/blood-products/document-migration/antivenomglrevwho_trs_1004_web_annex_5.pdf?sfvrsn=ef4b2aa5_3&download=true. 3. Data on file. BTG International Inc. 4. Tasoulis T, Isbister GK. Toxins (Basel). 2017;9(9):290. 5. Gutiérrez JM et al. [Erratum in: Nat Rev Dis Primers. 2017;3:17079]. Nat Rev Dis Primers. 2017;3:17063. 6. Strickland JL et al. Sci Rep. 2018;8(1):17622. 7. Riley BD et al. In: Nelson LS et al, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. McGraw-Hill Professional; 2010. 8. Borja M et al. Toxins (Basel). 2018;10(1):35.